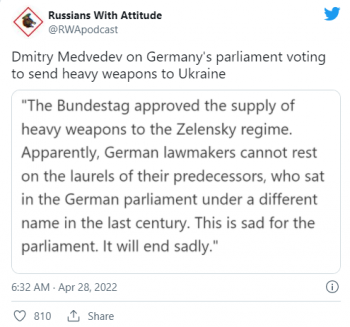
Moderna will ask FDA to approve its COVID vaccine for children SIX-MONTHS-OLD
by Daily Mail
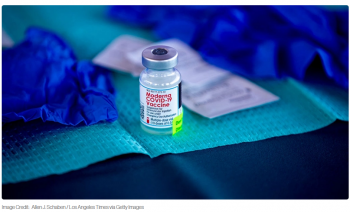
Moderna is planning to submit an application to the FDA on Thursday for the authorization of its COVID-19 vaccine among kids ages six months to five years.
The Omicron variant was predominant during Moderna’s pediatric trial, and the drugmaker claims two doses were around 38% effective in preventing infections in 2 to 5-year-olds and 44% effective for children aged 6 months to 2 years.
Moderna gave children two quarter-sized vaccine doses for the study. Their shot is currently only available for adults aged 18 and up. Pfizer’s COVID shot is available to children as young as five, and is the only vaccine authorized for minors in the US.
Its request comes after drug company suggested two-doses of its vaccine provided a comparable protection in young children to that of adults.
A clinical trial of around 6,700 kids found two quarter doses of the COVID-19 shot in children aged 6 and under generated an immune response similar to that of young adults who also received two full shots.
Many parents remain wary of giving young children the vaccine. They’re at very low risk of a severe COVID infection, with 1,185 of America’s total 992,000 COVID deaths among children aged 0 to 18. COVID jabs also carry a very low risk of heart inflammation – particularly in boys and young men – which can in rare cases prove serious.
by Daily Mail

Moderna is planning to submit an application to the FDA on Thursday for the authorization of its COVID-19 vaccine among kids ages six months to five years.
The Omicron variant was predominant during Moderna’s pediatric trial, and the drugmaker claims two doses were around 38% effective in preventing infections in 2 to 5-year-olds and 44% effective for children aged 6 months to 2 years.
Moderna gave children two quarter-sized vaccine doses for the study. Their shot is currently only available for adults aged 18 and up. Pfizer’s COVID shot is available to children as young as five, and is the only vaccine authorized for minors in the US.
Its request comes after drug company suggested two-doses of its vaccine provided a comparable protection in young children to that of adults.
A clinical trial of around 6,700 kids found two quarter doses of the COVID-19 shot in children aged 6 and under generated an immune response similar to that of young adults who also received two full shots.
Many parents remain wary of giving young children the vaccine. They’re at very low risk of a severe COVID infection, with 1,185 of America’s total 992,000 COVID deaths among children aged 0 to 18. COVID jabs also carry a very low risk of heart inflammation – particularly in boys and young men – which can in rare cases prove serious.